Getty/David Greedy
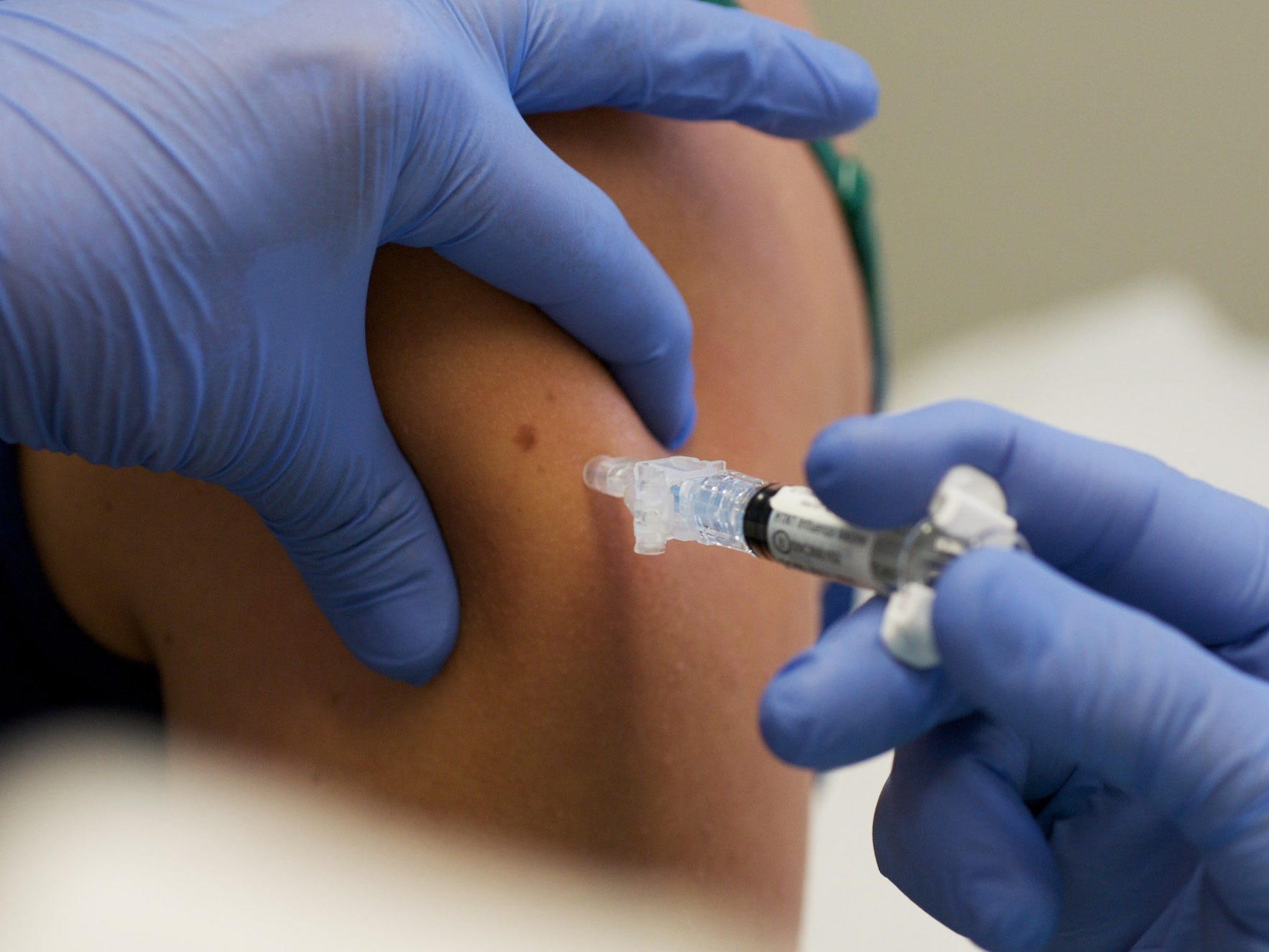
- Pfizer started testing the COVID-19 in children as young as six months.
- The 144 trial participants will receive varying doses.
- The trial will expand to 4,500 kids after researchers determine an effective dosage.
- Visit Insider's homepage for more stories.
Pfizer has launched its clinical trial for the COVID-19 vaccine, including children aged six months to 11 years old.
First in line were twin nine-year-old girls who received their first doses on Wednesday at Duke University.
The trial is an extension of Pfizer's ongoing study testing the vaccine in children aged 12 to 15 years old.
"We were encouraged by the data from the 12 to 15 group," Sharon Castillo, a spokeswoman for Pfizer, told the New York Times, prompting the company to begin the trial in children earlier than they initially said.
Pfizer joins Moderna and AstraZeneca in testing the COVID-19 vaccine in children. Moderna began a trial this month, and AstraZeneca in February.
The children will receive varying doses
The small trial will test three different doses (10, 20, and 30 micrograms) in 144 children across the globe. Adults are given two 3o microgram doses 21 days apart.
Researchers will split the participants into three age groups - five to 11 years old, two to four years old, and six months to two years old - and note the side effects and level of antibodies generated from each dose.
After picking the safest and most effective dosage, the trial will extend to 4,500 kids - two-thirds will the vaccine and one-third will receive the placebo.
Results for this trial aren't expected until after the second half of this year.