- The first drug developed to treat alopecia areata has shown promising results in clinical trials.
- Alopecia areata is the second-most common form of hair loss, affecting 2% of people.
- Multiple treatment options will be up for FDA approval soon.
An experimental drug restored a nearly full head of hair for some people with the second most common type of alopecia, according to clinical trial results shared Monday.
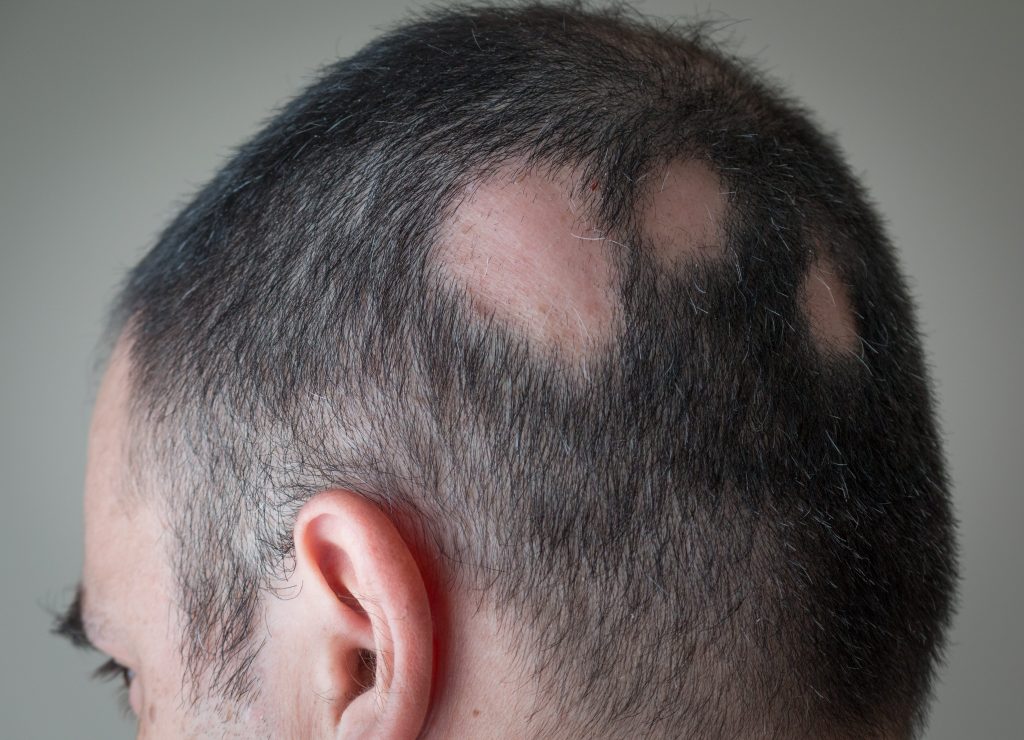
Alopecia areata is a skin disease that causes hair loss in about 2% of the population by recent estimates, or more than 6 million people in the US at some point in their lives.
The disease directs a person's immune system to attack their hair follicles, causing hair to fall out entirely or in patches. Hair follicles can grow back naturally over months or years, but there's no medication approved to treat the autoimmune disorder.
Concert Pharmaceuticals, a small company based in Lexington, Massachusetts, is one of a few drugmakers working on treatments for the disease.
Results from the first of two Phase 3 clinical trials of Concert's twice-daily pill, called CTP-543, showed significant hair regrowth in about 30–40% of volunteers who got the drug, according to a press release. The trial included a medium-dose and high-dose group, as well as a group who got an inactive placebo.
In both of the experimental groups, more than a quarter of participants saw at least 80% hair coverage by the end of the 24-week study, Concert Pharmaceuticals said.
"Eighty percent is a lot. I'd take that," alopecia advocate Thea Chassin, founder of Bald Girls Do Lunch, who was not involved in the clinical trial, told Insider. "It's very important to recognize that some hair growth, if it was a lower number like 20 or 30%, that's some hair but it's not a hairdo."
Some of the best data for alopecia areata treatment to date
If the results from Concert's second Phase 3 trial are also positive, the drug could be eligible for approval by the Food and Drug Administration in 2023. By then, people with alopecia may have other options for treatment.
Eli Lilly and Company, a much larger player in the pharmaceutical industry, is expected to request approval of its own drug to treat alopecia areata in the coming weeks, dermatologist and hair loss specialist Maryanne Senna wrote in an email to Insider.
The company has reported similarly positive results from Phase 3 trials of baricitinib, a pill currently approved for treatment of rheumatoid arthritis. Like alopecia areata, RA occurs when immune cells begin attacking healthy cells: joints in the case of arthritis, or follicles in alopecia.
In severe cases, people with alopecia areata may lose hair on their bodies and faces — including eyelashes and nose hairs — as well as their heads. Chassin, who is completely bald, said the disease can also affect healthy nail growth, leading to painful split nails.
Chassin told Insider that her experience with the disease has made her more confident in herself and her style, as she's grown a collection of hats, wigs, and hair wraps. But even the possibility of treatment could help ease the mental toll that comes with hair loss, she said.
"You're looking at yourself, and you don't recognize yourself, and this can lead to anxiety and depression," Chassin said. "And then the second whammy is, your dermatologist often will say, 'I don't have anything to offer you.' So there was no hope."
Senna, who has treated patients and led research into hair loss treatments, said the psychological and social impacts of these new medications cannot be underestimated.
"When my patients and study subjects regrow their hair because of these treatments, I see their smiles return," Senna said. "They tell me, 'I feel like I have my life back, I feel like myself again.'"