Joe Raedle/Getty Images
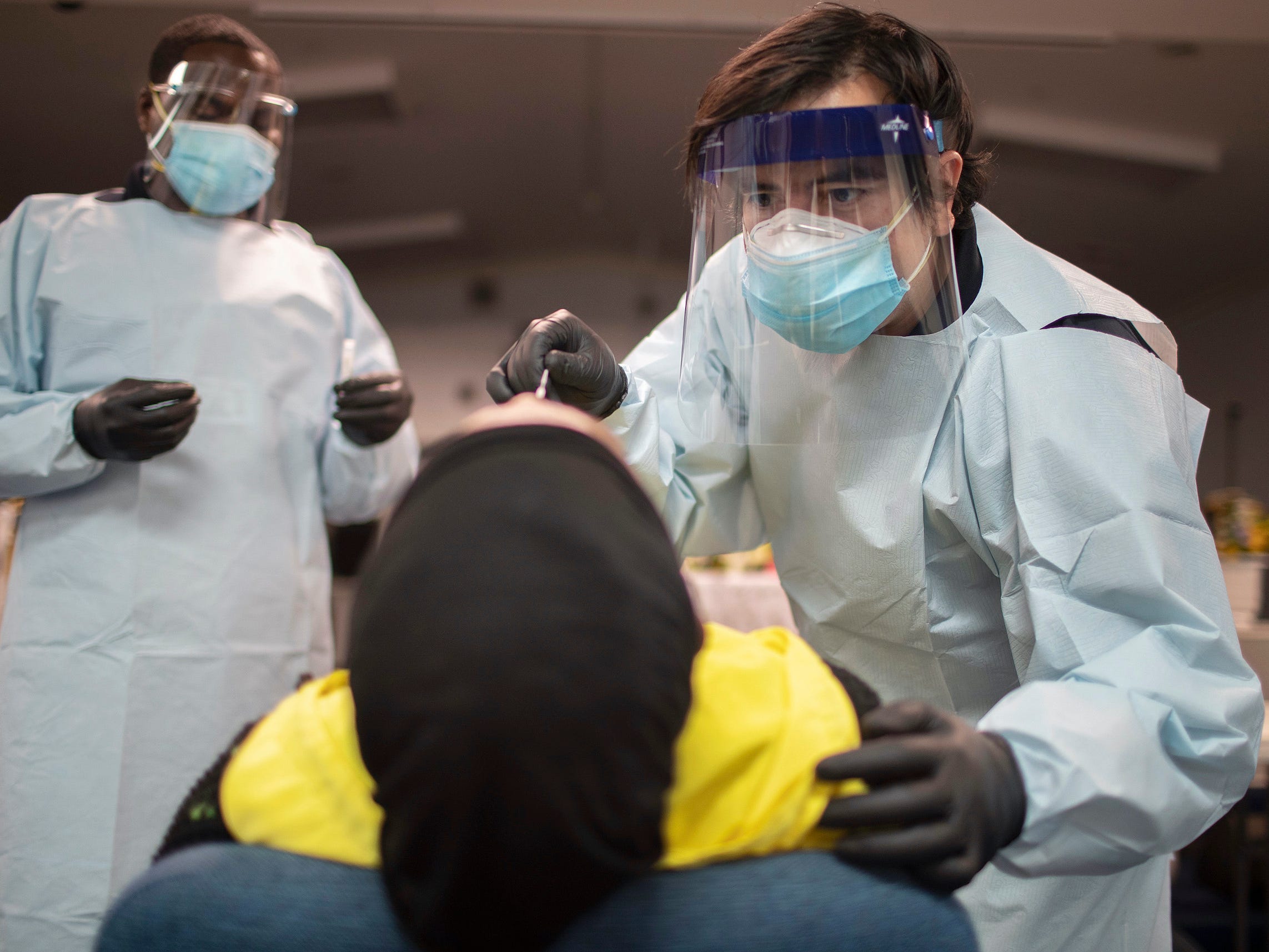
- States were still short of personal protective equipment, COVID-19 tests, and other vital medical supplies nine months into the pandemic, the Government Accountability Office (GAO) said in a report Monday.
- Half of states who responded to a survey by the watchdog in mid-October said they were short of rapid COVID-19 testing kits.
- Around the same number said they expected to have shortages over the next two months.
- Not being able to carry out enough tests means the disease could spread further, the GAO said.
- More than three-quarters of states expressed concerns about having supplies to distribute and administer a COVID-19 vaccine.
- Visit Business Insider’s homepage for more stories.
Half of all US states are worried they’ll run short of COVID-19 tests, according to a government watchdog report.
Some of the 47 states surveyed by the watchdog said they were still short of personal protective equipment (PPE) nine months into the pandemic, and more than three-quarters of states were concerned about having enough supplies to distribute and administer a COVID-19 vaccine.
The Government Accountability Office (GAO) urged the Department of Health and Human Services (HHS) to take urgent action in a report on the US’s pandemic response released Monday. It warned that not being able to carry out enough tests means the virus could spread further.
Around half of states said they were experiencing shortages of rapid testing kits in an mid-October survey by the GAO, which got responses from public health officials in 47 US states and territories.
Almost half said they didn’t have enough reagents — chemicals added to produce test results — and one third were experiencing shortages of other testing equipment.
Many of the states surveyed said they expected these problems to persist in the future. Half the states surveyed said they expected rapid test shortages in the 60 days following the survey, and 20 expected shortages of reagents.
"Testing supply shortages have contributed to delays in turnaround times for testing results, which can in turn exacerbate outbreaks by allowing COVID-19 to spread undetected," the GAO said.
The watchdog also criticized the Centers for Disease Prevention and Control's (CDC) changing guidelines on testing over the course of the pandemic "with little scientific explanation of the rationale behind the changes."
This "rais[es] the risk of confusion and eroding trust in important federal partners," the GAO said.
PPE shortages
Most of the respondents said their states had enough PPE, but there were supply problems in some categories, such as nitrile gloves and boot covers.
This varied greatly between states. For example, when asked about their ability to fulfill future requests for nitrile gloves, 17 states said they were "greatly" or "completely confident," while 15 states said they were only "slightly" confident or "not at all confident."
More than half the states who responded to the mid-October survey said they had obtained PPE supplies from either the commercial market or the Federal Emergency Management Agency (FEMA) in the past 30 days, which suggested they did not have adequate supplies to hand, the GAO said.
Almost three-quarters of states reported having obtained PPE from FEMA at some point during the pandemic.
"[This] indicates challenges in procuring these supplies from the commercial market, as states would only request supplies from FEMA when they were unable to meet their needs through the commercial market," the GAO said.
Vaccine concerns
In addition, more than three-quarters of states expressed concerns about having adequate supplies to distribute and administer a COVID-19 vaccine. One-third of the 47 respondents who said they were "greatly" or "completely concerned."
Officials from six states cited specific concerns about the federal government's ability to supply needles, given reports of shortages, and three of these states said this had impacted their flu vaccination efforts.
These concerns come as Pfizer, Moderna, and AstraZeneca marked major milestones in the global race for a vaccine in November. Pfizer's shot could get authorized in the US as early as December 10.
It isn't just a lack of needles that officials are worried about.
Hospitals around the US are panic-buying hyper-cold freezers in anticipation of Pfizer's vaccine, which must be kept at -94 degrees Fahrenheit to maintain its efficacy.
Experts told Business Insider they are also worried about a global shortage of glass vials to store the vaccines in.
"Highly successful" supply chain response
In response to the report, HHS told the GAO that, in cooperation with its federal partners, it has launched "the most comprehensive supply management effort undertaken by our nation since World War II."
The Administration has been "highly successful" in identifying and filling supply gaps across the US, it added.
HHS is unable to evaluate the claims because the GAO refused to identify the states who reported or expected shortages, it said.