University of Maryland
- Europe’s drugs regulator has fast-tracked the approval process for a COVID-19 vaccine developed by Germany’s BioNTech and US pharmaceutical group Pfizer.
- The “rolling review” by the European Medicines Agency (EMA) will potentially speed up the authorization of the vaccine.
- It means the EMA will not have to wait for trials to finish before reviewing data.
- The decision to begin the review is based on the results of earlier clinical trials that show the vaccine triggers an immune response in humans, the EMA said on Tuesday.
- Visit Business Insider’s homepage for more stories.
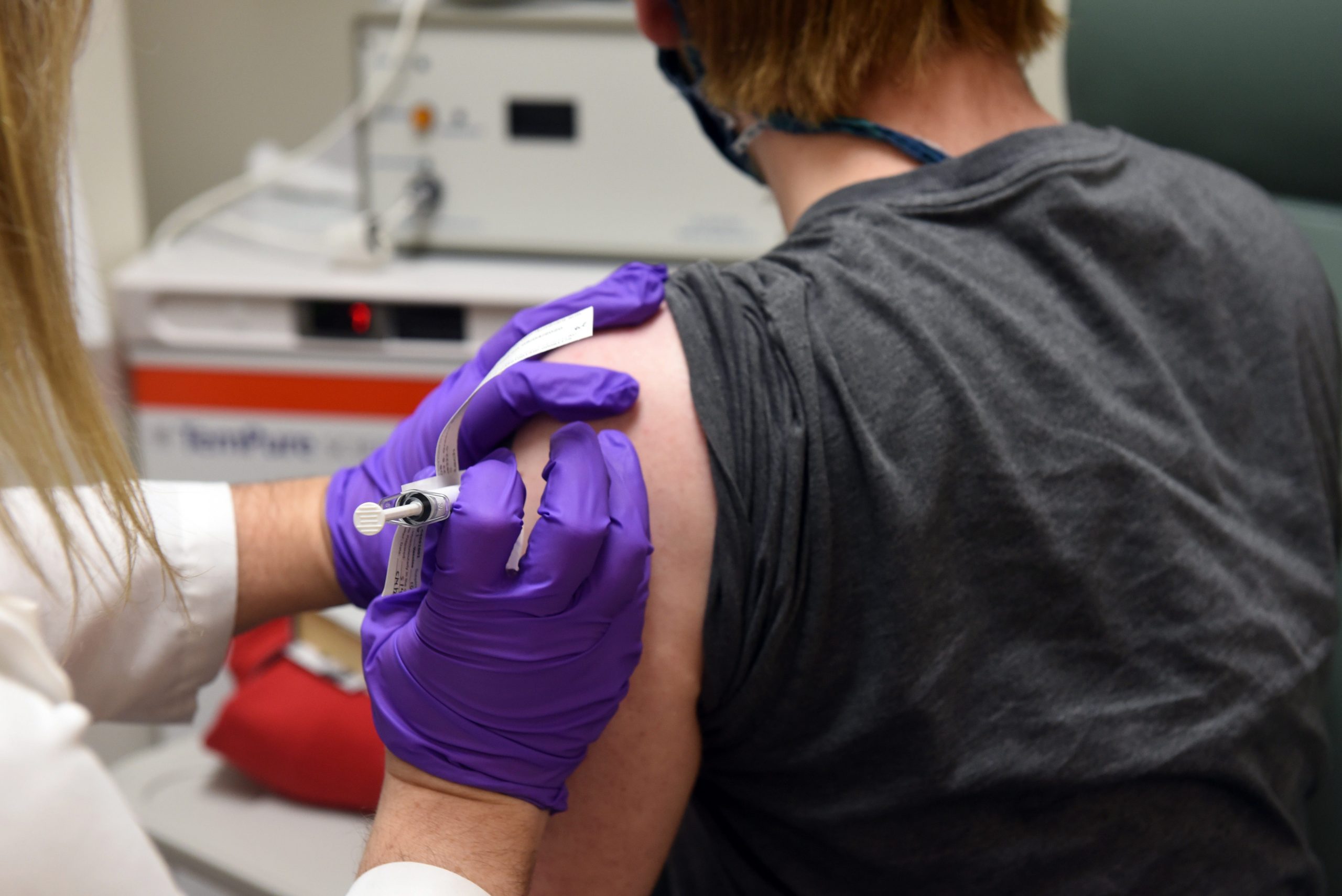
Europe’s drug regulator has sped up the potential approval of a COVID-19 vaccine developed by Germany’s BioNTech and US pharmaceutical group Pfizer.
A new “rolling review” launched by the European Medicines Agency (EMA) on Tuesday will evaluate how effective the vaccine is in real time as data returns from patient trails. This means the EMA will not have to wait for BioNTech and Pfizer to finish their trials and submit all the data at once.
The review could therefore speed up the authorization of the vaccine.
The EMA said on Tuesday it was looking at the first batch of data on the vaccine. It would continue to assess data until it had enough to make a final decision, it said.
The decision to kick off the rolling review of BioNTech and Pfizer’s COVID-19 vaccine is based on the results of earlier clinical trials in adults that showed the vaccine triggers an immune response, the EMA said.
The vaccine is the second COVID-19 candidate to be approved for a rolling review by the EMA. The agency confirmed on October 1 that it had started a rolling review of the vaccine developed by AstraZeneca and the University of Oxford. This followed a pause in trials of the vaccine, after a participant reported severe neurological symptoms — the UK has resumed trials, while the US is still investigating.
Pfizer's vaccine is currently in late-stage studies in the US, Brazil, South Africa, and Argentina.
BioNTech and Pfizer's vaccine could be one of the first COVID-19 candidates to be approved in Europe. The race for a vaccine on the continent is becoming ever more urgent as the infection rate rises.
The global coronavirus death toll passed 1 million on September 29.