- The preliminary results of Pfizer’s coronavirus vaccine trial are promising, but there are plenty of caveats to the good news.
- We still don’t know whether the vaccine protects against asymptomatic cases or if it even lowers the risk of spreading COVID-19.
- Questions of who will have access to the shot and when depend on how Pfizer and US public health agencies allocate the vaccine.
- Complete data on the vaccine’s effectiveness and safety won’t be publicly available until later this month.
- Visit Business Insider’s homepage for more stories.
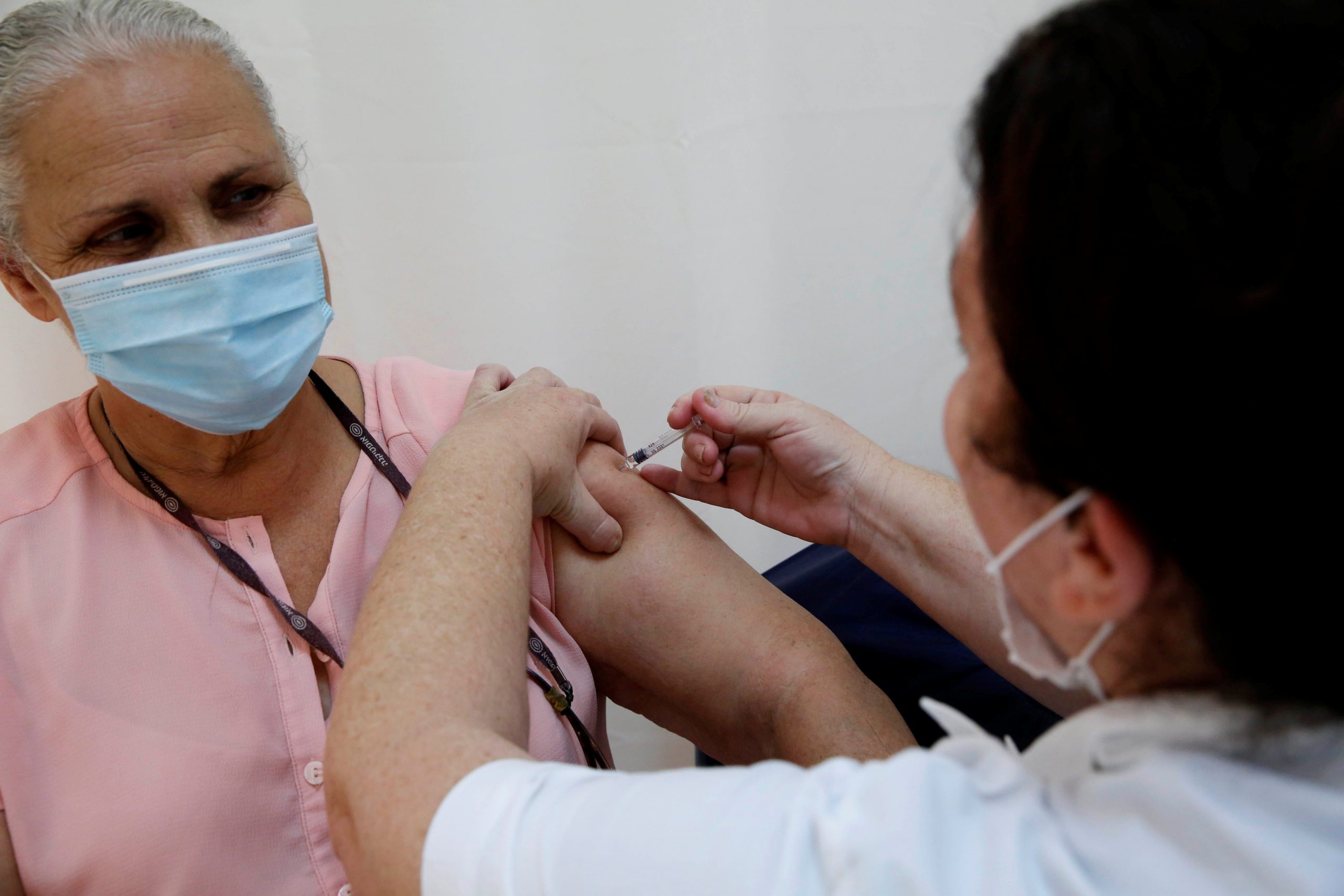
The pharmaceutical company Pfizer released a promising first look at their coronavirus vaccine trial results Monday, but many questions remain unanswered.
The drugmaker developed the vaccine in record time, and said it found the shots may be more than 90% effective at preventing COVID-19, the disease the novel coronavirus causes.
Experts have lauded the development, with the World Health Organization’s director general calling it an encouraging “unprecedented scientific innovation” and infectious-disease expert Dr. Anthony Fauci saying its efficacy rate is “remarkable.”
But scientists are also cautioning to understand that the news comes with plenty of caveats, as the trial is ongoing and the results not yet published. Plus, only 94 of the nearly 44,000 participants contracted COVID-19 over the course of the trial, and it’s unclear how many of them were given the vaccine versus the placebo.
Here are seven questions that need to be answered before Pfizer’s coronavirus vaccine goes to market.
Does the vaccine protect against both severe and mild illness?
To test the vaccine, Pfizer gave 43,538 volunteers either two doses of the vaccine or two salt-water placebo injections. An independent board of experts found that those who did end up contracting COVID-19 were far more likely to have received the sham shots.
But the study only considered people to have COVID-19 if they both tested positive and experienced symptoms, which means it's unclear if the vaccine also protects against asymptomatic cases.
We also don't know whether the vaccine reduces the risk of severe COVID-19 cases, hospitalizations, or death, Maria Elena Bottazzi, a co-director of Texas Children's Hospital Center for Vaccine Development, told Business Insider.
Can the vaccine stop virus transmission?
While the vaccine seems to lower the risk of contracting symptomatic COVID-19, we don't yet know if it lowers the risk of spreading the disease.
If, for instance, it keeps the person taking it from feeling sick or testing positive, but doesn't eliminate the contagious particles from their spit, they could be walking around unknowingly transmitting the illness to vulnerable people.
"The moment you get a vaccine doesn't mean you're going to put your mask in the trash," Bottazzi told Business Insider. "That is not going to happen. I hope people don't think that is going to be the magic solution for all.
How long will it be protective?
The trial found the vaccine became effective 28 days after the initial dose, but only looked at how effective it remained a week after getting the second dose. While Pfizer may continue following participants if and when emergency authorization is granted, we won't know if the vaccine confers long-term immunity for years.
"What will be the protective efficacy over time?" Gregory Poland, director of the Mayo Clinic's vaccine-research group, said, according to the Wall Street Journal. "Is this going to be a handful of months, like the flu vaccine? Is it going to be like measles or smallpox where it's lifelong immunity?"
What's the timeline for rolling out the vaccine?
Once Pfizer has more data about the safety of the vaccine later this month, the company plans to apply for an emergency use authorization from the Food and Drug Administration, which will fast-track vaccine approval and production.
Pfizer is on track to produce 50 million vaccine doses in 2020 and up to 1.3 billion doses in 2021. The drugmaker hasn't yet determined how those doses will be allocated across countries.
"Even if we got all 50 million, that's not going to solve the pandemic," Daniel Salmon, director of the Institute for Vaccine Safety at Johns Hopkins University, told Business Insider. "That's one-sixth, one-seventh of the population. And you're looking at a herd immunity threshold of maybe 60 or 70%."
Roll-out could hit a snag in parts of Asia, Latin America, and "all but a tiny corner of Africa" due to a shortage of cold storage facilities required to keep the vaccine at a viable temperature throughout the distribution process.
When will Americans be able to get vaccinated?
That's the million-dollar question, Salmon said. He and other experts have said that they're waiting to see more data before jumping to conclusions about when life will return to normal.
High-risk healthcare workers and first responders should be the first in line to receive the vaccine when it's available, according to a recommendation from the National Academies of Sciences. The first phase of the plan includes vaccinating 15% of the population, also prioritizing those with two or more preexisting conditions and elderly people living in congregate care settings.
Phases 2, 3, and 4 of the plan involve rolling out the vaccine to Americans in public-facing essential jobs, such as those working in schools and grocery stores, and to older adults with risk factors who didn't make the first group.
Ashish Jha, dean of Brown University School of Public Health, told NBC News it will take some time to get the vaccine to the American people. Healthcare workers could get vaccinated as early as January or February, he said, but the rest of us will likely have to wait to receive the shot between March and June 2021.
Will other vaccines work too?
While Pfizer and its German partner BioNTech are the first to announce positive results from a COVID-19 vaccine trial, other clinical trials by Moderna, AstraZeneca, and Johnson & Johnson are still underway.
The preliminary success of the Pfizer shot bodes well for results to come. All of the vaccine candidates are designed to target the same part of the coronavirus: the spike protein that allows it to attach to and invade host cells.
Dr. Anthony Fauci, the nation's leading disease expert, told STAT Pfizer's early success suggests that other vaccine trials are on the right track. Moderna's trial is especially likely to have promising results because it uses the same cutting-edge mRNA mechanism as Pfizer.
Moderna, a frontrunner in the vaccine effort, is on track to share its own results this month.
How safe is the vaccine, really?
Pfizer did not report any serious safety concerns in their recent press release, but the company is still in the process of gathering two months of safety data to submit to the FDA. After the data are under review, information about common, short-term side effects will be public knowledge.
However, no one will know the potential long-term side effects for a while.
Salmon said there's a chance that the eventual widespread vaccine rollout could result in some rare but severe reactions, so there needs to be a plan for addressing misinformation surrounding the vaccine's safety.
"There's a larger potential that if you vaccinate a lot of people quickly, a lot of bad things will happen to those people just by chance alone," he said. "If you vaccinate 30 million people over 65, you're going to have heart attacks and strokes within a day after vaccination. You need a system in place to separate coincidence from causal."